PLANT GROWTH-PROMOTING RHIZOBACTERIA AS PLANT BIOSTIMULANTS TO IMPROVE MINERAL NUTRITION AND RESOURCE USE EFFICIENCY
By: Youry Pii Faculty of Science and Technology, Free University of Bozen, Italy
email: youry.pii@unibz.it

An increase in crop productivity, while applying sustainable practices, is the main challenge for modern agriculture to satisfy the requests of the ever-growing world population in need of higher food quality and food security. Starting with the Green Revolution, the quality and the yields of agricultural productions have been improved by enhancing the application of chemical fertilizers and pesticides, impacting economic and environmental sustainability.
However, through the last decades, agricultural productivity has reached a stagnation phase in which yields have not proportionally increased with the amount of fertilizers applied, highlighting the need for improving resources use efficiency by crops (Pii et al., 2015a). When plants’ mineral nutrition is considered, research needs to focus on a specific soil volume, the rhizosphere – characterized by very close interactions between roots, microorganisms, and the soil itself (Hinsinger et al., 2009). In fact, the relationships established in the rhizosphere play a central role in modulating the bioavailability of mineral nutrients. Plants release root exudates, including low and high molecular weight organic compounds, that are involved, among other functions, in the mobilization of mineral nutrients from soil particles (Vives-Peris et al., 2020). However, low molecular weight organic compounds can also function as a strong chemoattractant for soil bacteria that can use them as an easily accessible carbon source (Hinsinger et al., 2009). The majority of rhizosphere bacteria (rhizobacteria) are commensals that take advantage of root exudates as nourishment.
On the other hand, pathogenic bacteria might establish negative interactions at rhizosphere levels, thereby producing toxic metabolites that have detrimental effects on plant growth. Differently, a group of microorganisms, known as Plant Growth-Promoting Rhizobacteria (PGPR), can exert a biostimulant action on plants through different mechanisms. PGPR can protect plants from pathogens, for example by activating plants’ defence systems or by stimulating the synthesis of stress-related compounds. In addition, PGPR can synthesize bioactive compounds, like hormones, which can promote plants growth with a higher accumulation of biomass, both above and below-ground (Crecchio et al., 2018). Indeed, the enhanced growth of the root system induced by PGPR allows plants to explore a higher volume of soil and ensure a higher surface for the interception of mineral nutrients, possibly resulting in an improved mineral nutrition provision. Nonetheless, in the recent decade, several pieces of evidence have highlighted that PGPR might also act on the molecular and biochemical mechanisms underlying nutrient acquisition processes in plants, allowing plants to better exploit the natural resources already present in soil (Pii et al., 2015b, 2015a, 2016).
Iron as a case study
Iron (Fe) is an essential element to plants, and it is fairly abundant on the Earth’s crust; nevertheless, its low bioavailability in soils often represents one of the major constraints to agricultural productivity (Kobayashi and Nishizawa, 2012). To face the issue of acquiring Fe from soils, plants have evolved different strategies (Astolfi et al., 2020; Marschner and Römheld, 1994); dicots and non-graminaceous monocots, for instance, i) acidify the rhizosphere by extruding protons through the activity of plasma membrane H+-ATPase, ii) reduce the insoluble FeIII to the more soluble FeII, via the activity of a ferric chelate reductase (FRO), and iii) transport FeII into root cells by the iron-regulated transporter 1 (IRT1) (Kobayashi and Nishizawa, 2012). These biochemical activities are strongly upregulated when plants are grown in soils featuring a very scarce Fe availability (Brumbarova et al., 2015), yet this is often not enough to prevent the appearance of Fe deficiency symptoms. Interestingly, several reports have highlighted that the contribution provided by PGPR dwelling in the rhizosphere might be crucial to assuring adequate Fe nutrition to plants (de Santiago et al., 2013; Pii et al., 2015a; Zhang et al., 2009; Zhao et al., 2014).
It was recently demonstrated that the inoculation of Fe deficient cucumber plants, grown in calcareous soils, with the PGPR Azospirillum brasilense led to a significant modification in the qualitative and quantitative pattern of root exudates release that eventually resulted in a quicker recovery from the nutritional disorder (Pii et al., 2015b). These observations further strengthened the idea that PGPR might improve plant mineral nutrition by affecting biochemical and molecular pathways. A deeper investigation of the mechanisms triggered by A. brasilense in Fe starved cucumber plants demonstrated that microorganisms could affect both the growth and architecture of the root system and plants’ ability to absorb Fe (Pii et al., 2016). The assessment of root morphology demonstrated that PGPR were able to enhance root growth, yet differently, according to the plant’s nutritional status: in Fe deficient plants, A. brasilense promoted the elongation of pre-existing lateral roots, while, in the case of Fe sufficient plants, the initiation of new lateral roots was triggered (Figure 1). At the molecular level, inoculated Fe deficient plants upregulated the genes encoding for the plasma membrane H+-ATPase and the ferric chelate reductase (FRO) as compared to control plants that allowed a more efficient (4-fold higher rate) Fe uptake (Pii et al., 2016). Interestingly, the inoculation with the PGPR also enhanced the Fe reduction activity in Fe sufficient plants, even though the gene expression analyses suggested that different molecular entities might be activated in this case (Marastoni et al., 2019; Pii et al., 2016). Consistently, genome-wide transcriptomic analyses showed that highly different sets of genes were modulated by the inoculation with the PGPR, depending on plants’ Fe provision, further corroborating that plant response to microbe interaction can be strongly dependent on the whole nutritional status.
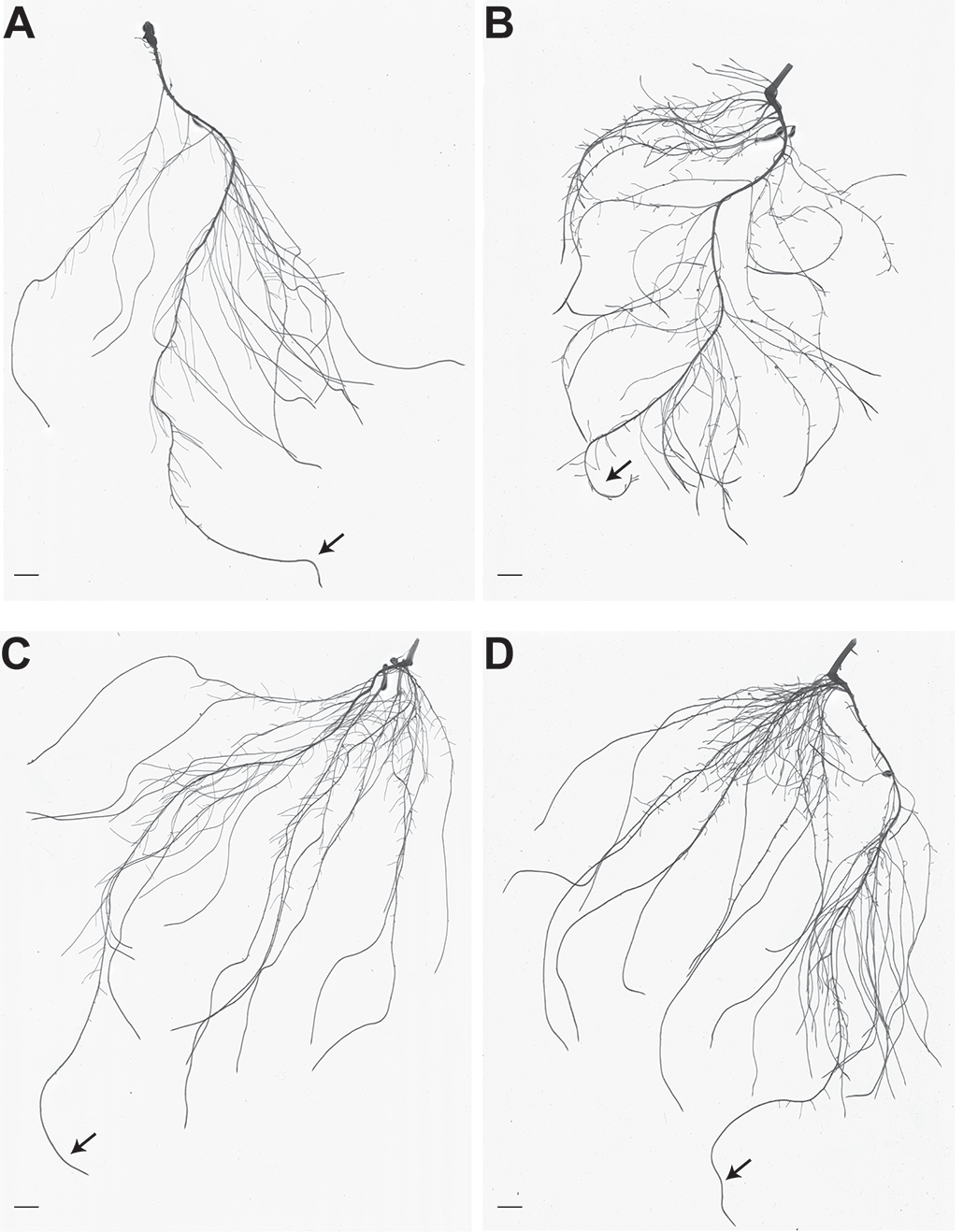
Figure 1. Root architecture of Fe sufficient and Fe deficient cucumber plants, either non-inoculated or inoculated with A. brasilense. A. Representative picture of the root system of cucumber plants grown in Fe-sufficient nutrient solution. B. Representative picture of the root system of cucumber plants grown in Fe-deficient nutrient solution. C. Representative picture of the root system of cucumber plants grown in Fe-sufficient nutrient solution and inoculated with A. brasilense. D. Representative picture of the root system of cucumber plants grown in Fe-deficient nutrient solution and inoculated with A. brasilense. Bar scales represent 1 cm. Arrows indicate primary roots. (Figure modified from Pii et al., 2016)
In conclusion, these pieces of evidence demonstrate that the use of PGPR in agriculture might improve the ability of plants to exploit the natural resources already available in the soil, allowing a reduction in the application of mineral fertilizers and an increase in agriculture sustainability. Moreover, very recent studies have demonstrated that, besides improving mineral nutrition, the use of PGPR as plant biostimulants can also positively affect the quality and the nutraceutical values of agricultural products (Kolega et al., 2020; Pii et al., 2018).
Astolfi, S., Pii, Y., Mimmo, T., Lucini, L., Miras-moreno, M. B., Coppa, E., et al. (2020). Single and Combined Fe and S Deficiency Differentially Modulate Root Exudate Composition in Tomato : A Double Strategy for Fe Acquisition ? Int. J. Mol. Sci., 21(11), 4038. doi:10.3390/ijms21114038.
Brumbarova, T., Bauer, P., and Ivanov, R. (2015). Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 20, 124–133. doi:http://dx.doi.org/10.1016/j.tplants.2014.11.004.
Crecchio, C., Mimmo, T., Bulgarelli, D., Pertot, I., Pii, Y., Perazzolli, M., et al. (2018). “Beneficial Soil Microbiome for Sustainable Agriculture Production,” in, ed. E. Lichtfouse (Cham: Springer International Publishing), 443–481. doi:10.1007/978-3-319-94232-2_9.
de Santiago, A., García-López, A. M., Quintero, J. M., Avilés, M., and Delgado, A. (2013). Effect of Trichoderma asperellum strain T34 and glucose addition on iron nutrition in cucumber grown on calcareous soils. Soil Biol. Biochem. 57, 598–605. doi:10.1016/j.soilbio.2012.06.020.
Hinsinger, P., Bengough, A. G., Vetterlein, D., and Young, I. (2009). Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321, 117–152. doi:10.1007/s11104-008-9885-9.
Kobayashi, T., and Nishizawa, N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 63, 131–52. doi:10.1146/annurev-arplant-042811-105522.
Kolega, S., Moreno, B. M., Buffagni, V., Lucini, L., Valentinuzzi, F., Maver, M., et al. (2020). Nutraceutical profiles of two hydroponically grown sweet basil cultivars as affected by the composition of the nutrient solution and the inoculation with Azospirillum brasilense. Front. Plant Sci. 11, 1683. doi:10.3389/FPLS.2020.596000.
Marastoni, L., Pii, Y., Maver, M., Valentinuzzi, F., Cesco, S., and Mimmo, T. (2019). Role of Azospirillum brasilense in triggering different Fe chelate reductase enzymes in cucumber plants subjected to both nutrient deficiency and toxicity. Plant Physiol. Biochem. 136, 118–126. doi:10.1016/j.plaphy.2019.01.013.
Marschner, H., and Römheld, V. (1994). Strategies of plants for acquisition of iron. Plant Soil 165, 261–274. doi:10.1007/BF00008069.
Pii, Y., Graf, H., Valentinuzzi, F., Cesco, S., and Mimmo, T. (2018). The effects of plant growth-promoting rhizobacteria (PGPR) on the growth and quality of strawberries. Acta Hortic., 231–238. doi:10.17660/actahortic.2018.1217.29.
Pii, Y., Marastoni, L., Springeth, C., Fontanella, M. C., Beone, G. M., Cesco, S., et al. (2016). Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ. Exp. Bot. 130, 216–225. doi:10.1016/j.envexpbot.2016.06.011.
Pii, Y., Mimmo, T., Tomasi, N., Terzano, R., Cesco, S., and Crecchio, C. (2015a). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 51, 403–415. doi:10.1007/s00374-015-0996-1.
Pii, Y., Penn, A., Terzano, R., Crecchio, C., Mimmo, T., and Cesco, S. (2015b). Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiol. Biochem. 87, 45–52. doi:10.1016/j.plaphy.2014.12.014.
Vives-Peris, V., de Ollas, C., Gómez-Cadenas, A., and Pérez-Clemente, R. M. (2020). Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep. 39, 3–17. doi:10.1007/s00299-019-02447-5.
Zhang, H., Sun, Y., Xie, X., Kim, M.-S., Dowd, S. E., and Paré, P. W. (2009). A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 58, 568–577. doi:10.1111/j.1365-313X.2009.03803.x.
Zhao, L., Wang, F., Zhang, Y., and Zhang, J. (2014). Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 54, S115–S124. doi:10.1002/jobm.201400148.



