Animal- versus plant-derived protein hydrolysates: different composition and mechanisms of action
By: Giovanna Marta Fusco, Rosalinda Nicastro, Pasqualina Woodrow, Petronia Carillo*
Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania, “Luigi Vanvitelli”, 81100 Caserta, Italy
*Email: petronia.carillo@unicampania.it

Animal and plant protein hydrolysates (A-PHs and V-PHs) obtained through incomplete hydrolysis of animal or vegetable residual biomasses and/or waste are new circular economy product categories for agriculture able to safe and sustainable boost food quality and yield (Calvo et al., 2014). PHs are mainly constituted by polypeptides, oligopeptides and amino acids (Calvo et al., 2014; Colla et al., 2015), carbohydrates, phenols, phytohormones, other organic compounds and trace minerals (Colla et al., 2015; Ertani et al., 2014). Their manufacturing processes require incomplete thermal, enzymatic, or chemical (alkaline or acid hydrolysis) partial hydrolysis of protein-rich animal waste and vegetable biomass sources (Calvo et al., 2014; Colla et al., 2015; du Jardin, 2015). PHs composition varies according to the animal or plant source of proteins and/or the manufacturing processes (Colla et al., 2017b; Ertani et al., 2009; Ertani et al., 2013). Once obtained, they may be used by root drenching, foliar application, and seed priming (Colla et al., 2015; Sorrentino et al., 2021).
A-PHs mostly derive from slaughterhouse by-products, through chemical hydrolysis of collagen at high temperatures (e.g., 100 °C). Notwithstanding the ethical and food safety concerns, they currently represent about 90% of PHs available on the market for their proven capacity to increase nitrogen use efficiency (NUE), plant growth and natural plant defences, improving the tolerance to salt stress, water stress, extreme temperatures, and pathogens. One of the reason of their capacity to exert stress protecting effects may be related to their high concentration of thermostable amino acids like hydroxyproline, hydroxylysine, proline, alanine and glycine (Rouphael et al., 2021). Hydroxyproline and hydroxylysine are abundant constituents of animal collagen being responsible for its functional properties by increasing cross-linking (Amirrah et al., 2022). In plants, hydroxyproline takes part in the structure of the hydroxyproline-rich glycoproteins (HRGPs), whose oxidative phenolic-amino acids residues coupling products are essential for the strengthening of cell walls and resistance against pathogens (Deepak et al., 2010). Instead, proline is a ubiquitous compatible osmolyte and ROS scavenger, which plays a pivotal role in stabilizing macromolecules, membranes, and cellular redox potential under drought, salt and/or oxidative stress. In addition, it may modulate the expression of genes containing proline or hypo-osmolarity-responsive element (PRE) in their promoters, necessary for the expression of the enzyme proline dehydrogenase (PDH) (Carillo, 2018). However, A-PHs are beneficial and cost-effective only when provided at low doses, while high doses or repeated foliar treatments may be toxic (Liu and Lee, 2012). In fact, since the manufacturing process requires acids or alkalis, A-PHs contain high concentrations of chloride and other salts that may induce osmotic and/or ionic stress. Moreover, A-PH derived from chemical hydrolysis can contain a large amount of free amino acids in D-form which are considered of low agronomic value. The high contents of glycine in A-PHs, when supplied by root drench, may increase the activity of 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and oxidase (ACO) and, therefore, the content of ethylene, thus reducing root elongation and RUE (Han et al., 2018) and enhancing leaf senescence. Also proline, if supplied at high concentrations (≥ 10 mM), may exert negative effects such as the inhibition of elongation of hypocotyls in Arabidopsis (Hare et al., 2001), or the quick increase of chloride at toxic concentrations in tomato leaves (Hayat et al., 2012). Probably the negative effects caused by exogenous proline at high doses may depend on the feedback inhibition of the enzymes involved its biosynthesis in the cytosol. In fact, the synthesis of proline from glutamate itself is able to fine tune several developmental processes (e.g., embryo formation, root elongation, flowering time and pollen fertility) by an unknow hormone-independent mechanism (Trovato et al., 2018). As for proline, also hydroxyproline and glycine betaine may cause feedback inhibition of their own synthesis. Whereas, high contents of hydroxylysine, may inhibit the expression of the isoforms GS1 and GS2 of glutamine synthetase in pee (Leason et al., 1982) and maize leaves (Acaster and Weitzman, 1985). In addition, a study from Bernabei (2015), showed that the production process of A-PHs has a higher energy cost and a higher environmental impact (water consumption and carbon dioxide emission) than the production process of plant hydrolysates obtained by enzymatic hydrolysis (Bernabei, 2015). Rouphael et al. (2021) demonstrated that basil plants treated with high doses of a commercial A-PH accumulated high levels of Na, Cl and proline. Since the impossibility to synthetise further proline to cope with the high levels of toxic ions, the plants were forced to promote alternative metabolic pathways for synthetizing in particular GABA, which is able to act as osmolyte modulating the osmotic potential between cytosol and vacuole, ROS scavenger and cytoplasmic acidosis buffer (Rouphael et al., 2021). Indeed, this diverts carbon skeletons from growth reducing plant yield.
On the contrary, V-PHs foliar treatments, also at significantly higher doses than those suggested by manufacturers, have never caused phytotoxicity symptoms (Cerdán et al., 2009; Colla et al., 2014; Kim et al., 2019). In addition, to produce V-PHs may be also used protein-rich crop residues or agri-food industry by-products, which represent a sustainable and economical raw material which, if recycled, helps to reduce the environmental impact and production costs (Colla et al. 2017b; Carillo and Morrone 2017). Moreover, reusing plant derived biogenic waste avoiding its unnecessary landfilling is pivotal to meet the EU’s Green Deal circular economy goals (European Commission, 2019). The use of enzymatic proteolysis to obtain V-PHs allows applying processing parameters like neutral pH values and temperatures lower than 60 °C that avoid the decomposition of thermolabile amino acids (e.g., the primary amino acids asparagine, aspartate, glutamate, glutamine, and the essential amino acids arginine, glycine and histidine) (Colla et al., 2015). The latter amino acids together with the oligopeptides and polypeptides contained in V-PHs, have been proven able to promote rooting response (Ceccarelli et al., 2021), increase plant resources use efficiency (RUE) (Colla et al., 2017a; Rouphael et al., 2021; Rouphael and Colla, 2018), modulate nitrogen and carbon metabolic activities (Colla et al., 2015; Ertani et al., 2013), boost yield (Colla et al., 2017a; Rouphael and Colla, 2018; Sestili et al., 2018), improve nutritional and nutraceutical quality (Paul et al., 2019; Rouphael and Colla, 2018) and increase abiotic stress tolerance (Colla et al., 2017b; Ertani et al., 2013; Lucini et al., 2018; Sorrentino et al., 2021). Indeed, these effects cannot be ascribed to the V-PHs organic nitrogen supplied due to the extremely low doses used, which are not comparable with those contained in the common nitrogen fertilizers (Halpern et al. 2015). Moreover, it has been proven that the V-PHs effects on root and shoot growth and the plastic modulation of root system architecture (e.g., length, number, density and surface of lateral roots) may be due to the bioactive peptides that V-PHs contain, able to exert auxin-like and/or gibberellin-like phytohormone activities and act as signalling molecules (Colla et al., 2015; Ertani et al., 2009; Lucini et al., 2018). Indeed, the higher capacity of these modified roots to explore the rhizosphere, together with the ability of V-PHs peptides and amino acids to complex nutrients in the soil and increase microbial activity speed up nutrients availability for root uptake, enhance the plant RUE and growth capacity (Carillo et al., 2019a; Colla et al., 2017b). Besides, V-PHs, when supplied by root drenching, can replace the functions of synthetic iron chelators averting the risk of their high mobility in the soil profile (Cristofano et al., 2021). Glutamate, present at high concentration in V-PHs, may act as organic chelator improving iron uptake and promoting FeIII-chelate reductase activity both in roots and shoots, thus increasing the concentration of iron in leaves and photosynthetic activity especially in young seedlings (Cerdán et al., 2013; Jeong et al., 2008; Olanrewaju et al., 2019). In fact, the use of V-PHs has been shown able to improve iron (and in general ions) content, colour status, photosynthesis, production and quality in basil, spinach and perennial wall rocket leaves (Carillo et al., 2019b; Caruso et al., 2019; Rouphael et al., 2021). All these beneficial effects exerted by V-PHs might be described as “nutrient acquisition response” and result in a general improvement of uptake, translocation, and assimilation of nutrients (Carillo et al., 2019b; Cristofano et al., 2022; Sestili et al., 2018).
It is undeniable that both types of biostimulants are valid supplements to agricultural practices capable of boosting crop yield and produce quality. However, while for V-PHs there are no adverse effects, it is necessary to pay close attention to the dosage of A-PHs to avoid metabolism and growth negative disorders and optimize crop response (Figure 1)
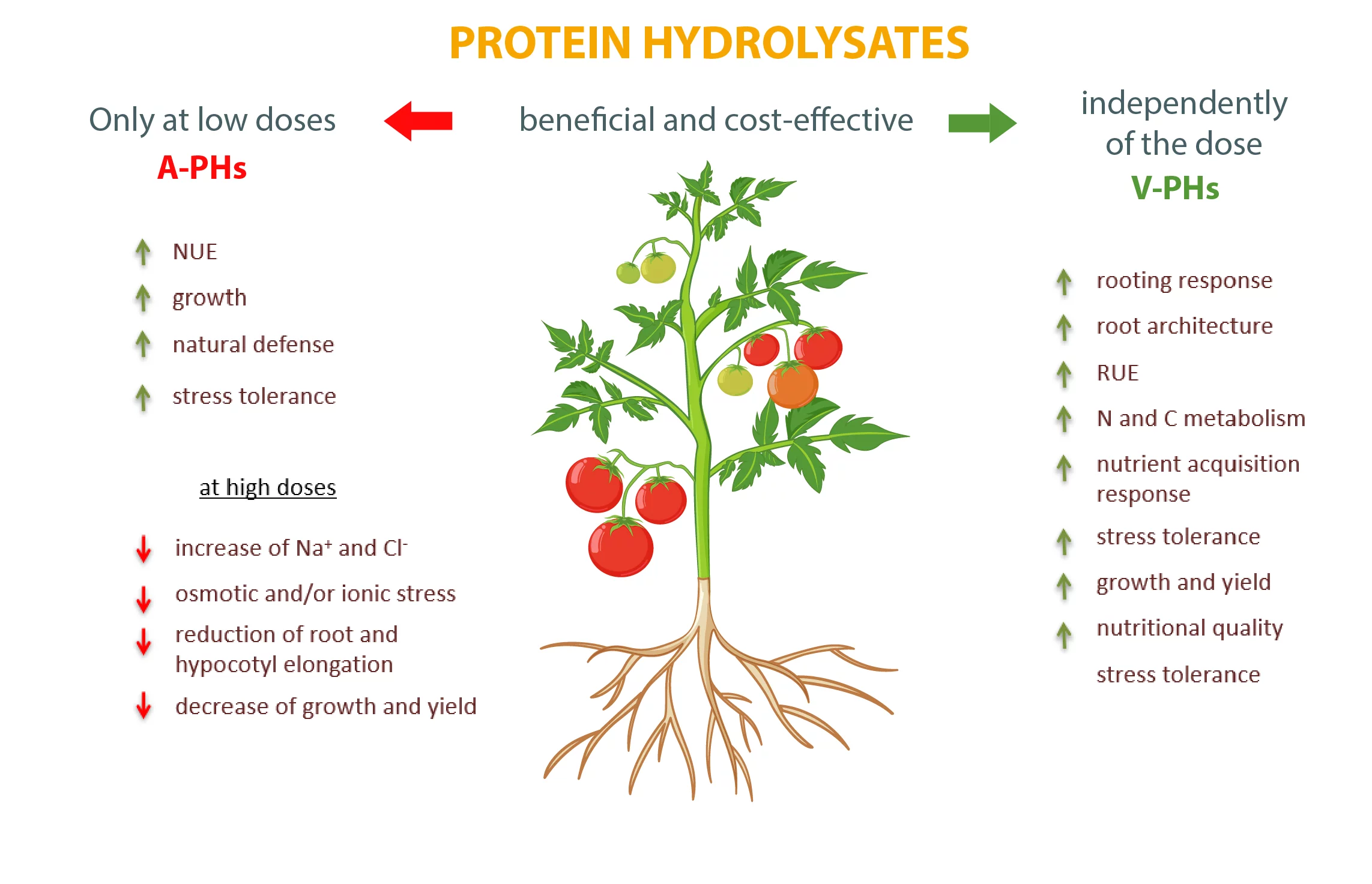
Fig. 1. Main dose-dependent effects exerted by animal and plant protein hydrolysates (A-PHs and V-PHs) on vegetable crops.
Acaster M.A., Weitzman P.D.J. (1985) Kinetic analysis of glutamine synthetases from various plants. FEBS Letters 189:241-244. DOI: 10.1016/0014-5793(85)81031-0.
Amirrah I.N., Lokanathan Y., Zulkiflee I., Wee M.F.M.R., Motta A., Fauzi M.B. (2022) A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 10:2307.
Calvo P., Nelson L., Kloepper J.W. (2014) Agricultural uses of plant biostimulants. Plant and Soil 383:3-41. DOI: 10.1007/s11104-014-2131-8.
Carillo P. (2018) GABA Shunt in Durum Wheat. Frontiers in Plant Science 9. DOI: 10.3389/fpls.2018.00100.
Carillo P., Colla G., El-Nakhel C., Bonini P., D’Amelia L., Dell’Aversana E., Pannico A., Giordano M., Sifola M.I., Kyriacou M.C., De Pascale S., Rouphael Y. (2019a) Biostimulant Application with a Tropical Plant Extract Enhances Corchorus olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 9:249.
Carillo P., Colla G., Fusco G.M., Dell’Aversana E., El-Nakhel C., Giordano M., Pannico A., Cozzolino E., Mori M., Reynaud H., Kyriacou M.C., Cardarelli M., Rouphael Y. (2019b) Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 9:450.
Caruso G., De Pascale S., Cozzolino E., Giordano M., El-Nakhel C., Cuciniello A., Cenvinzo V., Colla G., Rouphael Y. (2019) Protein Hydrolysate or Plant Extract-based Biostimulants Enhanced Yield and Quality Performances of Greenhouse Perennial Wall Rocket Grown in Different Seasons. Plants (Basel) 8. DOI: 10.3390/plants8070208.
Ceccarelli A.V., Miras-Moreno B., Buffagni V., Senizza B., Pii Y., Cardarelli M., Rouphael Y., Colla G., Lucini L. (2021) Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism, Plants.
Cerdán M., Sánchez-Sánchez A., Jordá J.D., Juárez M., Sánchez-Andreu J. (2013) Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. Journal of Plant Nutrition and Soil Science 176:859-866. DOI: 10.1002/jpln.201200525.
Cerdán M., Sánchez-Sánchez A., Oliver M., Juárez M., Sánchez-Andreu J.J. (2009) Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Acta Horticolturae 830:481-488.
Colla G., Cardarelli M., Bonini P., Rouphael Y. (2017a) Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 52:1214-1220. DOI: 10.21273/HORTSCI12200-17.
Colla G., Hoagland L., Ruzzi M., Cardarelli M., Bonini P., Canaguier R., Rouphael Y. (2017b) Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Frontiers in Plant Science 8. DOI: 10.3389/fpls.2017.02202.
Colla G., Nardi S., Cardarelli M., Ertani A., Lucini L., Canaguier R., Rouphael Y. (2015) Protein hydrolysates as biostimulants in horticulture. Scientia Horticulturae 196:28-38. DOI: https://doi.org/10.1016/j.scienta.2015.08.037.
Colla G., Rouphael Y., Canaguier R., Svecova E., Cardarelli M. (2014) Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Frontiers in Plant Science 5. DOI: 10.3389/fpls.2014.00448.
Cristofano F., El-Nakhel C., Colla G., Cardarelli M., Pii Y., Lucini L., Rouphael Y. (2022) Tracking the Biostimulatory Effect of Fractions from a Commercial Plant Protein Hydrolysate in Greenhouse-Grown Lettuce. Antioxidants (Basel) 12. DOI: 10.3390/antiox12010107.
Cristofano F., El-Nakhel C., Rouphael Y. (2021) Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species, Biomolecules.
Deepak S., Shailasree S., Kini R.K., Muck A., Mithöfer A., Shetty S.H. (2010) Hydroxyproline-rich Glycoproteins and Plant Defence. Journal of Phytopathology 158:585-593. DOI: https://doi.org/10.1111/j.1439-0434.2010.01669.x.
du Jardin P. (2015) Plant biostimulants: Definition, concept, main categories and regulation. Scientia Horticulturae 196:3-14. DOI: https://doi.org/10.1016/j.scienta.2015.09.021.
Ertani A., Cavani L., Pizzeghello D., Brandellero E., Altissimo A., Ciavatta C., Nardi S. (2009) Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. Journal of Plant Nutrition and Soil Science 172:237-244. DOI: 10.1002/jpln.200800174.
Ertani A., Pizzeghello D., Francioso O., Sambo P., Sanchez-Cortes S., Nardi S. (2014) Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: chemical and metabolomic approaches. Frontiers in plant science 5:375-375. DOI: 10.3389/fpls.2014.00375.
Ertani A., Schiavon M., Muscolo A., Nardi S. (2013) Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant and Soil 364:145-158. DOI: 10.1007/s11104-012-1335-z.
European Commission. (2019) A European Green Deal: Striving to Be the First Climate-Neutral Continent. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 5 June 2023).
Han R., Khalid M., Juan J., Huang D. (2018) Exogenous glycine inhibits root elongation and reduces nitrate-N uptake in pak choi (Brassica campestris ssp. Chinensis L.). PLOS ONE 13:e0204488. DOI: 10.1371/journal.pone.0204488.
Hare P.D., Cress W.A., van Staden J. (2001) The effects of exogenous proline and proline analogues on in vitro shoot organogenesis in Arabidopsis. Plant Growth Regulation 34:203-207. DOI: 10.1023/a:1013326526875.
Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. (2012) Role of proline under changing environments: a review. Plant signaling & behavior 7:1456-1466. DOI: 10.4161/psb.21949.
Jeong J., Cohu C., Kerkeb L., Pilon M., Connolly E.L., Guerinot M.L. (2008) Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions. Proc Natl Acad Sci U S A 105:10619-24. DOI: 10.1073/pnas.0708367105.
Kim H.-J., Ku K.-M., Choi S., Cardarelli M. (2019) Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes. Agronomy 9:74.
Leason M., Cunliffe D., Parkin D., Lea P.J., Miflin B.J. (1982) Inhibition of pea leaf glutamine synthetase by methionine sulphoximine, phosphinothricin and other glutamate analogues. Phytochemistry 21:855-857. DOI: https://doi.org/10.1016/0031-9422(82)80079-4.
Liu X.-Q., Lee K.-S. (2012) Effect of Mixed Amino Acids on Crop Growth, Agricultural Science, Dr. Godwin Aflakpui (Ed.), ISBN: 978-953-51-0567-1, InTech, Available from: http://www.intechopen.com/books/agricultural-science/effect-of-mixed-amino-acids-on-crop-growth.
Lucini L., Rouphael Y., Cardarelli M., Bonini P., Baffi C., Colla G. (2018) A Vegetal Biopolymer-Based Biostimulant Promoted Root Growth in Melon While Triggering Brassinosteroids and Stress-Related Compounds. Frontiers in plant science 9:472-472. DOI: 10.3389/fpls.2018.00472.
Olanrewaju O.S., Ayangbenro A.S., Glick B.R., Babalola O.O. (2019) Plant health: feedback effect of root exudates-rhizobiome interactions. Applied microbiology and biotechnology 103:1155-1166. DOI: 10.1007/s00253-018-9556-6.
Paul K., Sorrentino M., Lucini L., Rouphael Y., Cardarelli M., Bonini P., Reynaud H., Canaguier R., Trtílek M., Panzarová K., Colla G. (2019) Understanding the Biostimulant Action of Vegetal-Derived Protein Hydrolysates by High-Throughput Plant Phenotyping and Metabolomics: A Case Study on Tomato. Frontiers in Plant Science 10. DOI: 10.3389/fpls.2019.00047.
Rouphael Y., Carillo P., Cristofano F., Cardarelli M., Colla G. (2021) Effects of vegetal- versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Scientia Horticulturae 284:110123. DOI: https://doi.org/10.1016/j.scienta.2021.110123.
Rouphael Y., Colla G. (2018) Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Frontiers in Plant Science 9. DOI: 10.3389/fpls.2018.01655.
Sestili F., Rouphael Y., Cardarelli M., Pucci A., Bonini P., Canaguier R., Colla G. (2018) Protein Hydrolysate Stimulates Growth in Tomato Coupled With N-Dependent Gene Expression Involved in N Assimilation. Frontiers in Plant Science 9. DOI: 10.3389/fpls.2018.01233.
Sorrentino M., De Diego N., Ugena L., Spíchal L., Lucini L., Miras-Moreno B., Zhang L., Rouphael Y., Colla G., Panzarová K. (2021) Seed Priming With Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Frontiers in Plant Science 12. DOI: 10.3389/fpls.2021.626301.



