Current assessment of the effectiveness of biostimulants
By: Danny Geelen
HortiCell, Ghent University, Coupure links, 653, 9000 Gent, Belgium
*Correspondence: danny.geelen@ugent.be

Crop yield is the most important factor for food security worldwide and in many cases also determines the profitability of crop production. The impact of any condition that affects yield such as fertilization and climate conditions therefore have the attention of agricultural engineer and the farmer. While for many pests and diseases agrochemical products offer the means to limit yield losses, far fewer solutions are available for mitigating abiotic stresses. Biostimulants are viewed as an easy approach to protect crops from to severe damage, despite of a history of variable impact. To allow the biostimulant market to mature and provide products of better quality, filtering the good from the bad will be important. In this review paper, we limit our discussion to the non-microbial biostimulants.
Biostimulants come in different types from organic chemicals, specific mineral formulae (silicon, phosphite, …), and micro-organisms. This large variety of materials and organisms complicates defining what biostimulants are, which has led to various attempts reported over the last decade (du Jardin, 2015; Yakhin et al., 2017; Ricci et al., 2019). The definitions that likely will have the largest impact are those given by governmental bodies. The Council of the European Union* has prepared a directive in 2018 which becomes effective 16th July 2022 and in the US the USDA, EPA, and FDA are discussing the rules in place. Fortunately, the general tendency is to define biostimulants primarily by their bioactivity, paving the way for an array of products. Evidently, new products have to be safe for humans and environment.
Traditionally, however, biostimulants are categorized by the material of origin. A non exhaustive list of biostimulants includes seaweed extract (SWE), plant extracts (PE), chitosan (CH), humic and fulvic acid (HFA), and protein hydrolysates (PH) (du Jardin, 2015; Colla and Rouphael, 2015). This grouping is very useful for activity comparisons as one associates the products with a specific organic chemical group, with the exception of plant extracts for which much less information on chemical composition is available (Xu and Geelen, 2018). However, the bioactivities attributed to biostimulants are very diverse, suggesting that multiple active compounds are present (Yakhin et al., 2017). Seaweed for instance contains several chemicals to which biostimulant activity can be attributed (Carmody et al., 2020; Langowski et al., 2021). Most biostimulants are mixtures and show a diversity of reported beneficial properties, suggesting that determining the effectiveness of a biostimulant will be complex.
Determining how well biostimulants work will depend on what bioassay is used and how well the chemical composition was described. Analysis of both these factors is work in progress and requires more dedicated research for collecting the details. Attempts to assess the average effectiveness of biostimulants have been made based on what has appeared in the literature so far (Jing et al., 2022; Herrmann et al., 2022). These studies provide new insights into factors that influence the effectiveness of biostimulants, albeit interpretations should be made with caution as the analyses were done using published data for which we speculate there is a bias towards reporting primarily positive results. An upfront conclusion that can be drawn is that there is a need for publicizing also negative result from biostimulant use. Such data would be highly valuable in gaining insight into the conditions in which to apply biostimulants as well as identifying the parameters have a negative impact on the effectiveness.
In this context it is of interest to present how biostimulant and biopesticide results are organized and structured database (Fig. 1) generated in a project called Bio2Bio (Jing et al., 2022b and paper under review). Comparisons between different biostimulant products are ideally made by performing parallel treatments in a randomized block design (Hartung, et al., 2019). Under these conditions, the variation of external factors not controlled by the experiment is usually limited and comparisons more reliable. The number of biostimulants products on the market and the many screening experiments done by research labs globally do not allow such experiments to be set up. Alternative methods to execute comparisons are therefore required. In the Bio2Bio project, we performed 38 bioassays and screened 64 different extracts from biomass waste. To compare the activities of these extracts across the different bioassays, data were collected and organized using the software Tableau. The biomasses were analyzed for the presence of pesticide residues to dereplicate results and avoid identifying compounds using during crop production. The extracts were also tested for ecotoxicity as several biopesticide assays monitor the viability of pathogens. General toxins are effective yet rarely lead to valuable products as these products do not pass legislation. In parallel the extracts were subjected to various bioassays and raw data introduced in the Tableau software. Since every bioassay follows a unique scoring system, direct comparison of data is not possible. To address this issue, we normalized the data following a linear (max-min) equation and referred to this as the BBC index (Ugena et al., 2018).
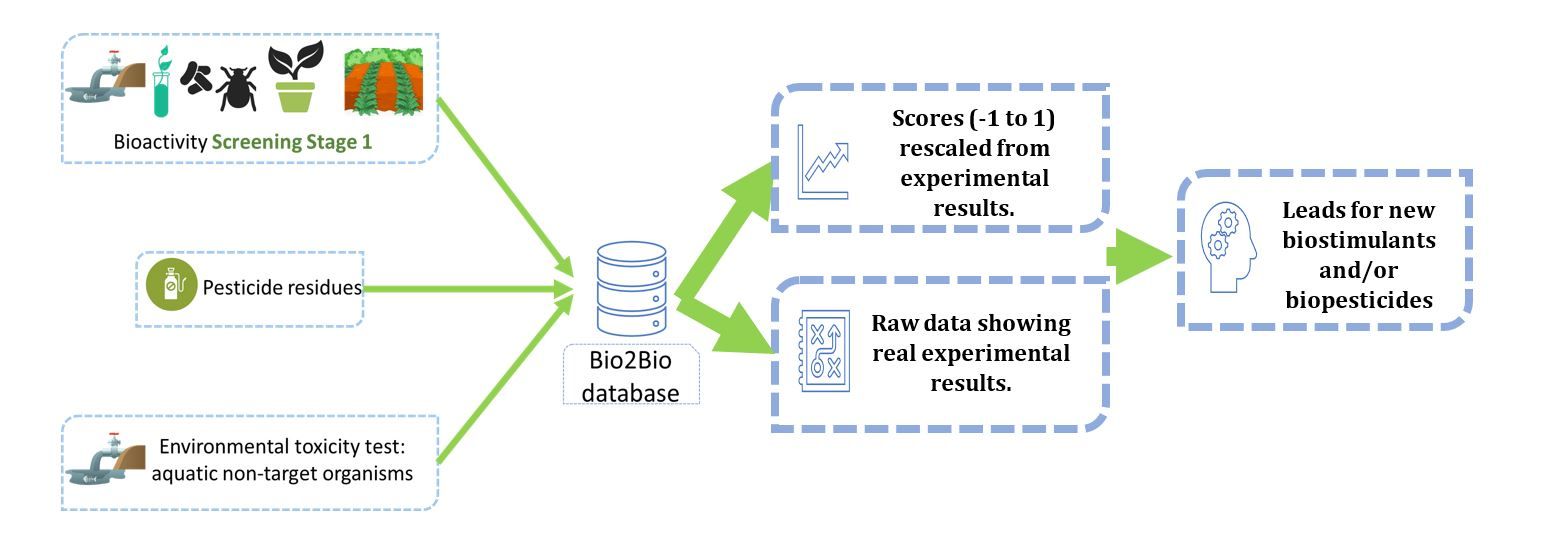
Fig. 1. Comparison of biostimulant and biopesticide activities using a normalized database.
The normalized data facilitate the visualization of the quantitative traits in a parallel coordinate histogram. Extract can be ranked according to performance or alternatively the trait performance is ranked for a specific extract. This allows a quick assessment of the different extracts across a large number of bioassays. A database setup like this can be developed for much larger datasets extrapolating the comparisons beyond the limits of a single experiment or that of an entire project. In a recently published paper, we compared the data from non-microbial biostimulants collected from over one thousand pairs of open-field data in a total of 180 studies worldwide (Jing et al., 2022). Data were collected from biostimulants containing one of the main bioactive substances: Chi, HFA, PHs, Phi, SWE, Si, and PE (Du Jardin, 2015; Rouphael and Colla, 2020). The experimental crop yield data were collected from the original tables or extracted from the attached figures using WebPlotDigitizer (Rohatgi, 2020). The impact of biostimulants on crop yield parameters was compared across 4 main groups of moderator variables including experimental-, plant-, climate-, and soil-related parameters. We organized the data also by application methods (foliar, soil, and seed treatment), by cultivated crop group (cereals, vegetables, fruits, legumes, root/tuber crops, and other crops) and by climate (main classes: equatorial, arid, warm temperate, and boreal; and 6 subclasses: desert, steppe, monsoonal, summer dry, winter dry, and fully humid). The meta-analysis revealed novel insights that do not immediately follow from published data as such. A surprising finding was that plant extracts were reported to show a higher average yield increase compared to the more conventional biostimulants SWE, CHI, HFA and PH. It is not clear why plant extracts show a higher effectiveness. There may be a bias in the available data as plant extracts are more recently developed and composed of a wider variety of biomass than the conventional biostimulants.
The yield increase was found to depend on the application method (foliar, seed, and soil) and associated variables (frequency, dose, and interannual application). Soil treatment, an indirect application method, resulted in the most substantial yield increase (+28.8%), Foliar application is more popular as this method is better amenable to a larger area of non-irrigated farmland. Foliar treatments yielded on average a 17.0% increase similar to seed applications, which is substantially lower than soil treatments. The lower impact is not compensated for by increasing the number of treatments. In fact, single and double spraying were about the same in effectiveness whereas more frequent applications were counterproductive. This result accords with the idea that biostimulants are genuine enhancers of cellular growth, different from mineral nutrients that are building blocks for growth rather than growth regulators.
The effectiveness of biostimulants was found to depend on the type of crop tested. Vegetable crops showed the highest yield benefit (+22.8%), differing by more than twofold from that of tuber crops (+10.6%). Legumes responded much better to biostimulant applications than fruits, cereals, and other crops. It is not clear what the physiological or biochemical basis is for these findings. It may be that vegetable crops have been bred more intensively, generating genotypes that are pushed to use much higher growth rates at the expense of reduced stress tolerance. Any small change in the growth condition may cause a greater yield penalty that can be suppressed by biostimulant application.
Finally, a comparison across 4 main climate categories (equatorial, arid, warm temperate, and boreal) and six precipitation types (desert, steppe, monsoonal, summer dry, winter dry, and fully humid) revealed that biostimulant effectiveness was most positive in climates with seriously limited water availability (arid and desert).
These findings show correlations between biostimulant effectiveness and impactors that have, insofar as we are aware, not previously been completely recognized. In general, biostimulants were mostly effective under sub optimal growth conditions of stress with limited water availability as an important parameter. A second important factor that was revealed is the importance of application frequency. To improve the effectiveness of biostimulants, it will be critical to invest in research that investigates the conditions of biostimulant application. What is the best growth stage of the crop, the ideal climate condition for treatment? The impact of biostimulant application on crop yield does therefore largely depend on acquired experience with a product that is accumulated over several years. Data collection of both positive and negative results will be a key component to evaluate the effectiveness of biostimulants.
Carmody N., Goñi O., Łangowski Ł., O’Connell S. (2020). Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 11:807. doi: 10.3389/fpls.2020.00807
Colla, G., Rouphael, Y. 2015. Biostimulants in horticulture. Sci. Hortic. 196, 1–2
*Council of the European Union (2018). Proposal for a Regulation of the European Parliament and of the Council Laying Down Rules on the Making Available on the Market of CE Marked Fertilizing Products and Amending Regulations (EC)No 1069/2009 and (EC) No 1107/2009-Analysis of the Final Compromise Text With a View to Agreement. Available at: http://data.consilium.europa.eu/doc/document/ST-15103-2018-INIT/en/pdf.
du Jardin P. (2015). Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 196, 3–14. doi: 10.1016/j.scienta.2015.09.021
Hartung, J., Wagener, J., Ruser, R. et al. Blocking and re-arrangement of pots in greenhouse experiments: which approach is more effective? Plant Methods 15, 143 (2019). https://doi.org/10.1186/s13007-019-0527-4
Jing Li, Thijs Van Gerrewey and Danny Geelen (2022a). A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Frontiers in Plant Science. doi: 10.3389/fpls.2022.836702.
Jing Li, Philippe Evon, Stephane Ballas, Hoang Khai Trinh, Lin Xu, Christof Van Poucke, Bart Van Droogenbroeck, Pierfrancesco Motti, Sven Mangelinckx, Aldana Ramirez, Thijs Van Gerrewey and Danny Geelen (2022b). Sunflower Bark Extract as a Biostimulant Suppresses Reactive Oxygen Species in Salt-Stressed Arabidopsis. (Frontiers in Plant Science, accepted).
Langowski Ł., Goñi O., Marques F. S., Hamawaki O. T., da Silva C. O., Nogueira A. P. O., et al.. (2021). Ascophyllum nodosum extract (SealicitTM) boosts soybean yield through reduction of pod shattering-related seed loss and enhanced seed production. Front. Plant Sci. 12:631768. doi: 10.3389/fpls.2021.631768
Rohatgi, A. (2020). Webplotdigitizer: Web Based Tool to Extract Data From Plots, Images, and Maps. Available online at: https://automeris.io/WebPlotDigitizer.
Ricci M., Tilbury L., Daridon B., Sukalac K. (2019). General principles to justify plant biostimulant claims. Front. Plant Sci. 10:494. doi: 10.3389/fpls.2019.00494
Ugena, L., Hýlová, A., Podlešáková, K., Humplík, J.F., Doležal, K., Diego, N. De, Spíchal, L., 2018. Characterization of biostimulant mode of action using novel multi-trait high-throughput screening of arabidopsis germination and rosette growth. Front. Plant Sci. 9. https://doi.org/10.3389/fpls.2018.01327
Yakhin O. I., Lubyanov A. A., Yakhin I. A., Brown P. H. (2017). Biostimulants in plant science: a global perspective. Front. Plant Sci. 7:2049. doi: 10.3389/FPLS.2016.02049.



